
OS X NECK DIAGRAMS TRIAL
We recognize that while crossover can bias OS results, the allowance and handling of crossover in trials should be limited to appropriate circumstances.Ĭrossover, when a patient in the control arm receives the interventional drug upon tumor progression, can introduce bias in oncology trials, especially since trial results are often interpreted with the intention-to-treat principle, where data are analyzed based on treatment assignment and not actual treatment receipt. Nineteen percent of RPSFT use is appropriate. RPSFT is a common tactic used by the industry to reinterpret trial results. The correlation between the uncorrected and corrected OS hazard ratio difference and the percentage of crossover was 0.44 (95% CI: 0.21 to 0.63). Twelve studies (19%) tested a drug’s fundamental efficacy when there was no SOC 34 studies (52%) tested a drug’s fundamental efficacy when there was already a SOC and 19 studies (29%) tested a drug’s sequential efficacy. All studies were funded by the industry or had authors who were employees of the industry. The median percentage of crossover was 56% (quartile 1, quartile 3: 37% to 72%). ResultsĪmong 65 studies, the median difference between the uncorrected and corrected OS hazard ratio was −0.1 (quartile 1, quartile 3 : −0.3 to −0.06). We calculated the percentage of RPSFT studies evaluating a drug for fundamental efficacy (with or without a standard of care (SOC)) or sequential efficacy and the correlation between the OS hazard ratio difference (unadjusted and adjusted) and the percentage of crossover. In a cross-sectional analysis (2003–2023), we reviewed oncology randomized trials that used RPSFT analysis to adjust the OS hazard ratio for patients who crossed over to an anti-cancer drug. We sought to examine the strength of correlation between differences in uncorrected and corrected OS hazard ratios and percentage of crossover, and characterize instances of fundamental and sequential efficacy.
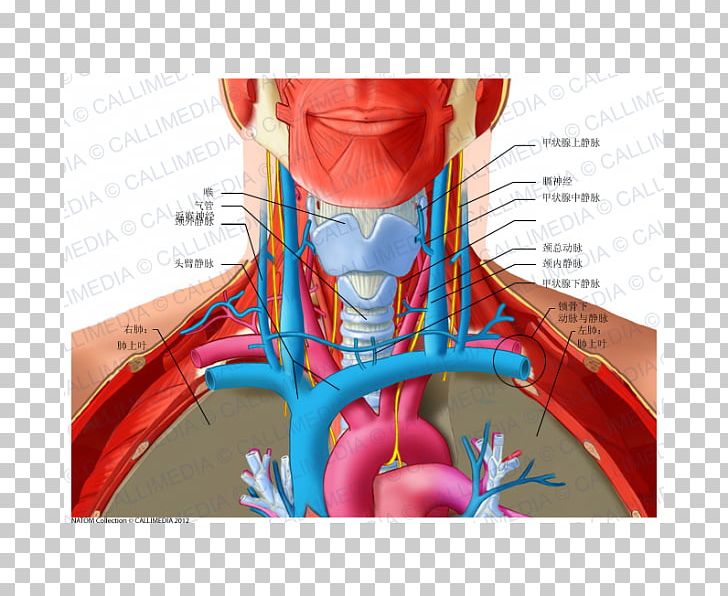
Rank preserving structural failure time (RPSFT) is a statistical method to correct or adjust for crossover in clinical trials, by estimating the counterfactual effect on overall survival (OS) when control arm patients do not receive the interventional drug when their tumor progresses.


 0 kommentar(er)
0 kommentar(er)
